Prevention
FDA Panel Says At-Home HIV Test is Safe, Effective
FDA Panel Says At-Home HIV Test is Safe, Effective
By continuing to use our site, you agree to our Private Policy and Terms of Use.
FDA Panel Says At-Home HIV Test is Safe, Effective
FDA Panel Says At-Home HIV Test is Safe, Effective

An independent Food and Drug Administration panel unanimously concluded Tuesday that an over-the-counter, at-home test would be safe and effective.
The panel of 17 advisors agreed that the test would encourage more people to know their status and therefore take preventative measures to ensure they don't spread the virus. According to Reuters, the panel's recommendation will soon be considered by agency regulators to determine whether OraSure will be available in stores and sold as the first at-home HIV testing kit available in the United States.
According to OraSure Technologies Inc., the test is expected to retail for less than $60 if approved and made available within the next few months.
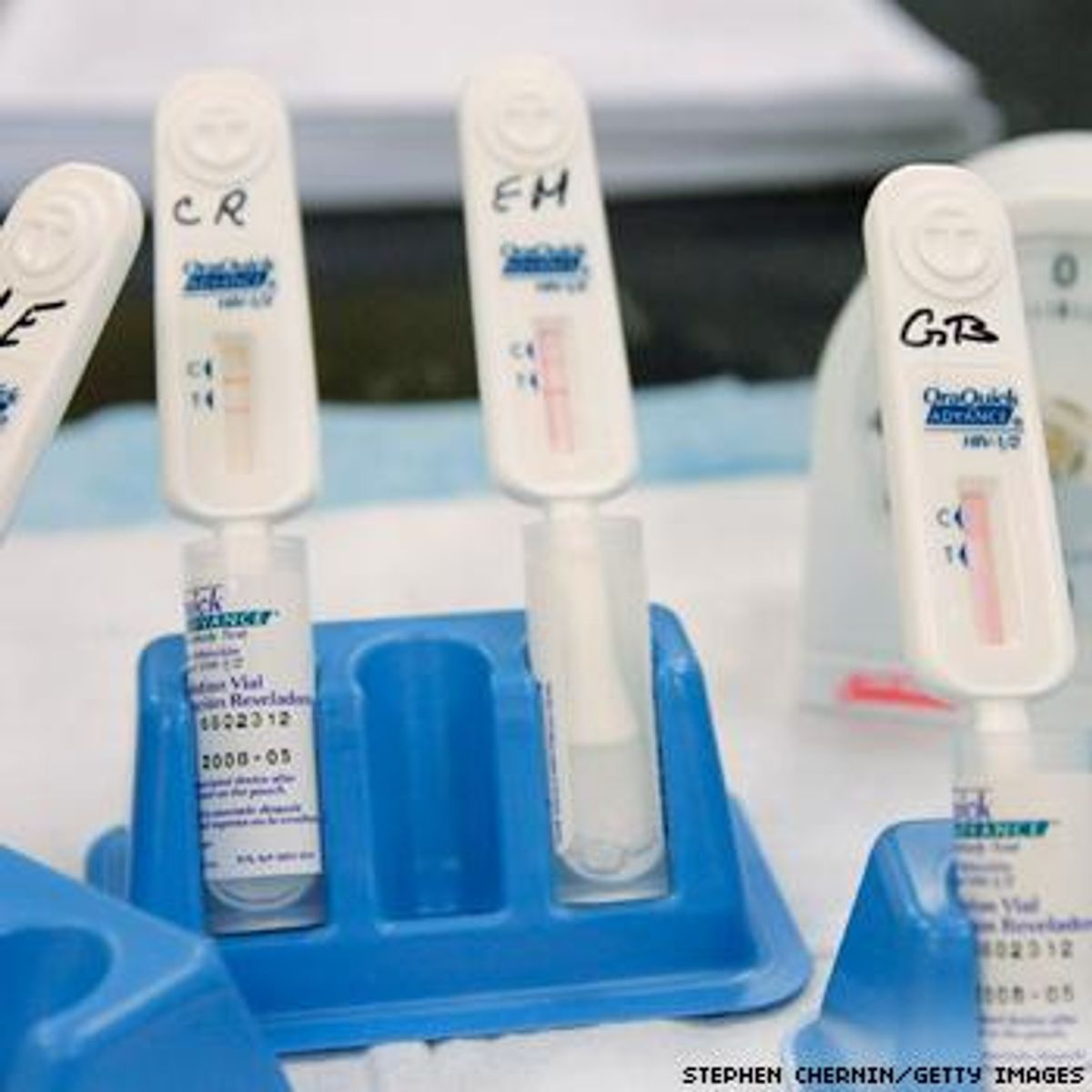
The new product is a version of the professionally administered OraQuick Advance test (pictured), an oral swab that provides results in 20 minutes.
"...One in five people living with HIV in America do not know it," said National Minority AIDS Council Director of Legislative and Public Affairs Kali Lindsey. "The OraQuick In-Home HIV Test would provide an important tool to supplement current HIV screening efforts by providing an accessible, relatively inexpensive device that can be used in the privacy of one’s own home."
Want more breaking equality news & trending entertainment stories?
Check out our NEW 24/7 streaming service: the Advocate Channel!
Download the Advocate Channel App for your mobile phone and your favorite streaming device!