Treatment GuideJust DiagnosedSex & DatingAfrican AmericanStigmaAsk the HIV DocPrEP En EspañolNewsVoicesPrint IssueVideoOut 100
CONTACTCAREER OPPORTUNITIESADVERTISE WITH USPRIVACY POLICYPRIVACY PREFERENCESTERMS OF USELEGAL NOTICE
© 2026 Pride Publishing Inc.
All Rights reserved
All Rights reserved
By continuing to use our site, you agree to our Privacy Policy and Terms of Use.
In the continuing effort to find ways to block HIV replication, researchers are unraveling more and more mechanisms that HIV uses to attach to a CD4 cell, gain entry, convert its RNA to DNA, and bud off a new virus. This is leading to an ever-growing list of new classes of drugs in the development pipeline. To understand one of the newest and most promising classes'entry inhibitors'new terms and tests need to be introduced. The first three classes of antiretrovirals'nucleosides, nonnucleosides, and protease inhibitors'worked within the CD4 cell to block steps in replicating HIV. Next came Fuzeon, which worked one step before the intracellular drugs'blocking the way the HIV membrane fuses with the CD4 membrane once the virus had attached to the CD4 cell. Now there are drugs trying to block an even earlier step at the entry coreceptor. When HIV approaches a T cell the proteins clustered on the surface of the virus attach to the CD4 molecule on the surface of the cell. The next step requires these viral proteins to bind to a chemokine receptor, or entry molecule, on the T cell. Then the membranes of the virus and the T cell are modified to allow fusion. There are two chemokine receptors'CCR5 and CXCR4 (abbreviated as R5 or X4)'and there are now drugs starting early trials that can block one or the other. But this is not all as simple as it sounds. About 80% of patients are infected with HIV that uses the R5 receptor, 20% use the X4 molecule, and about 1% are a mixture of R5 and X4. But there are big differences in the clinical course of people with R5 virus and those with X4 virus. People with R5 virus generally have higher CD4 counts and lower viral loads than those with X4 virus. They also have a slower rate of HIV progression. But as CD4-cell counts fall, there is a shift in the viral population toward X4 so that up to 50% of people with lower counts are X4. This has serious consequences, since X4 viruses can form clumps of CD4 cells called syncytia, which enable more rapid HIV destruction of those cells. Another negative of this shift is that R5 virus seems to attach only to mature primed CD4 cells, while the X4 molecule is found more commonly on immature cells in the thymus or newly activated CD4 cells. This means that X4 virus is destroying the cell line at its very beginning. Lots of questions need to be addressed. Most important, should we consider initiating HIV therapy before the R5-to-X4 shift starts? Some studies indicate that this shift may start in the 400 to 500 CD4-cell range. Second, will it be helpful to use an R5 blocker early to preclude a shift to X4 virus, or will it actually drive the virus to become X4? Will an X4 blocker be useful in salvage regimens? There is evidence that HIV can mutate to become resistant to X4, but will this mutated strain be less fit? Also there is some evidence that using an X4 blocker may cause the reemergence of the less aggressive R5 strain. Will this be helpful? There will soon be a commercially available test for R5 and X4, so maybe by 2007 we will have some of these drugs available and some of the questions answered. So in the future when your doctor has your lab results and tells you your viral load, CD4-cell count, genotype, and receptor molecule type, you will be a step ahead in knowing how the information might help you. Bowers is board-certified in family practice and is a senior partner with Pacific Oaks Medical Group, one of the nation's largest practices devoted to HIV care, located in Beverly Hills, Calif. He has served on the boards of AIDS Research Alliance and Lambda Legal. He is on the editorial board of Postgraduate Medicine.
From our Sponsors
Most Popular
“So much life to live”: Eric Nieves on thriving with HIV
September 03 2025 11:37 AM
The Talk: Beyond the exam room
August 13 2025 3:15 PM
Messenger RNA could be the key to an HIV vaccine — but government cuts pose a threat
August 20 2025 8:02 AM
It’s National PrEP Day! Learn the latest about HIV prevention
October 10 2025 9:00 AM
Amazing People of 2025: Javier Muñoz
October 17 2025 7:35 PM
The lab coat just got queer
August 21 2025 10:00 AM
The Talk: Owning your voice
August 25 2025 8:16 PM
“I am the steward of my ship”: John Gibson rewrites his HIV narrative
September 16 2025 2:56 PM
Plus: Featured Video
Latest Stories
HIV-positive men stage 'Kiss-In' protest at U.S.-Mexico border
December 01 2025 12:56 PM
What the AIDS crisis stole from Black gay men
December 01 2025 6:00 AM
The Talk: Navigating your treatment
August 01 2025 6:02 PM
The Talk: Starting the conversation
July 25 2025 4:47 PM
Thanks to U=U, HIV-positive people can live long, happy, healthy lives
July 25 2025 2:37 PM
How the Black AIDS Institute continues to fill in the gaps
July 25 2025 1:06 PM
“I felt like a butterfly”: Niko Flowers on reclaiming life with HIV
July 23 2025 12:22 PM
Dancer. Healer. Survivor. DéShaun Armbrister is all of the above
July 02 2025 8:23 PM
BREAKING: Supreme Court rules to save free access to preventive care, including PrEP
June 27 2025 10:32 AM
1985: the year the AIDS crisis finally broke through the silence
June 26 2025 11:24 AM
VIDEO: A man living with HIV discusses his journey to fatherhood
June 10 2025 4:58 PM
Trump admin guts $258 million in funding for HIV vaccine research
June 03 2025 3:47 PM
Grindr is reminding us why jockstraps are so sexy and iconic
May 02 2025 5:36 PM
HRC holds 'die-in' to protest Trump health care cuts
April 28 2025 2:11 PM
Two right-wing Supreme Court justices signal they may uphold access to PrEP and more
April 21 2025 4:10 PM
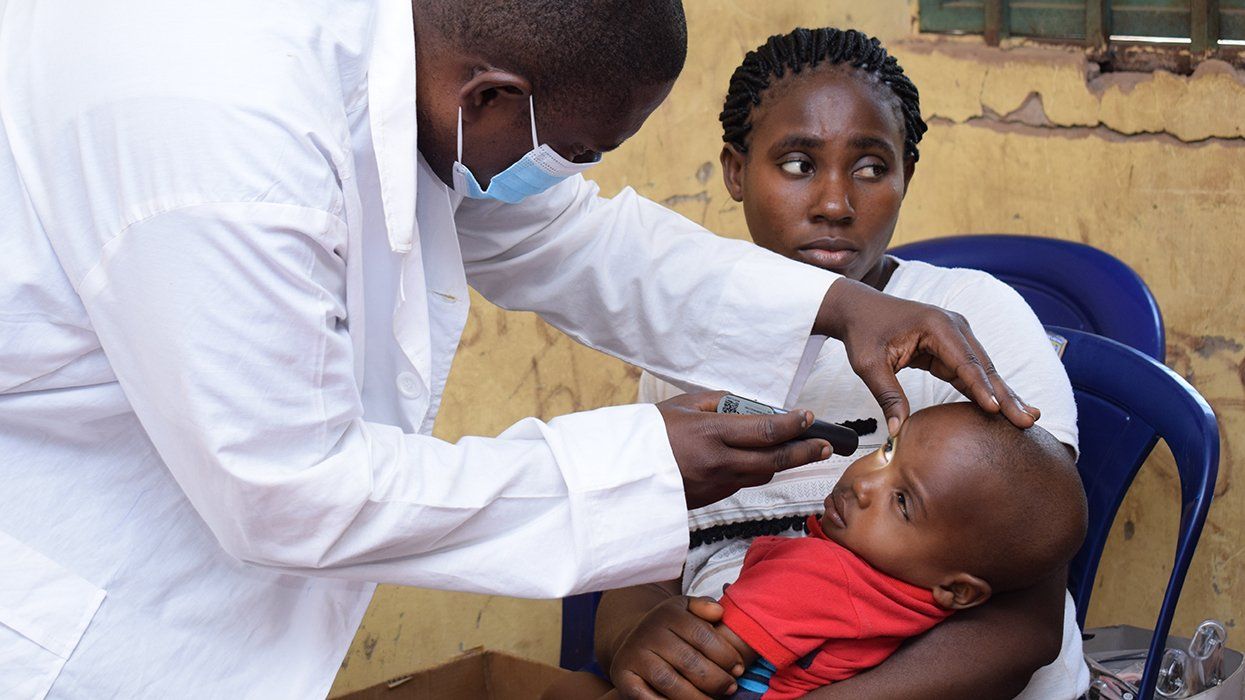
500,000 Children at Risk: PEPFAR Funding Crisis
April 08 2025 3:51 PM
Trending stories
Recommended Stories for You