Treatment GuideJust DiagnosedSex & DatingAfrican AmericanStigmaAsk the HIV DocPrEP En EspañolNewsVoicesPrint IssueVideoOut 100
CONTACTCAREER OPPORTUNITIESADVERTISE WITH USPRIVACY POLICYPRIVACY PREFERENCESTERMS OF USELEGAL NOTICE
© 2025 Pride Publishing Inc.
All Rights reserved
All Rights reserved
Scroll To Top
By continuing to use our site, you agree to our Private Policy and Terms of Use.
After the approval of three new antiretrovirals'two in new medication classes'within the past year, the slowdown in the drug pipeline for the foreseeable future might seem disheartening. But researchers haven't taken a break from their efforts to make new, viable anti-HIV meds. In fact, in recent months their efforts have led to discoveries about the immune system that will very likely open new frontiers for attacking the virus. One such discovery could take the number of viable antiretroviral classes far beyond the current half a dozen. While HIV has to hijack human proteins that are part of CD4 cells to do its damage, scientists have known only a few dozen such targets. But Harvard researchers have unveiled a surprisingly longer list'an important first step in the hunt for new meds. On its face HIV is a simple virus, consisting of just nine genes. Yet it makes up for that bare-bones structure in a sinister and complex way'by literally taking over the cellular machinery of its victims so that it can multiply and then destroy. The proteins it exploits have been dubbed HIV dependency factors, and 36 had been discovered. The new research, published online in the journal Science, found 273 of these potential targets. Led by geneticist Stephen Elledge of Brigham and Women's Hospital, the team used a technique called RNA interference that can disrupt a gene's ability to do its job and make a protein. One by one, they disrupted thousands of human genes in test tubes, dropped in some HIV, and watched what happened. If HIV couldn't grow well, it signaled the protein that the gene that had failed to produce must be the reason. It will take more research to figure out the role each of these proteins plays in HIV's life cycle, Elledge says. But most of today's anti-HIV meds work by targeting the virus itself. In August, the government approved sale of the first drug that works by blocking one of HIV's dependency factors, a cellular doorway called CCR5. The hope is that this longer list of those factors will point toward spots where similar drugs might work. And other research has experts hoping that they've uncovered an all-new way to defeat the virus. By outfitting immune-system killer cells with a new pair of genes, scientists have transformed them into weapons that destroy cells infected with HIV. Their strategy of genetically engineering immune cells to redirect their infection-fighting ability toward killing HIV-infected cells could lead to an entirely new approach for combating the virus, according to their study, reported in the March issue of the Journal of Virology. After someone is infected with HIV, a subgroup of their immune cells known as CD8 cytotoxic T lymphocytes recognize cells infected with HIV and kill them before they become HIV-producing factories. This CD8 activity initially keeps the infection in check. But eventually the virus typically evades and ultimately overpowers the immune system. However, a small percentage of HIVers, known as elite controllers, manage to suppress their infection for years. 'Certain of the CD8s of elite controllers may be genetically equipped to bind tightly to HIV-infected cells and destroy them and thereby suppress the infection indefinitely,' says Harris Goldstein, MD, senior author of the study and director of the Einstein/Montefiore Center for AIDS Research at Yeshiva University. 'Our idea,' he says, 'was first to identify the elite controllers' 'super' CD8s and to isolate the genes that enable these cells to bind tightly to HIV-infected cells and kill them efficiently; then we would transfer these genes into CD8s that do not recognize HIV-infected cells and convert them into potent killers of those cells.' The researchers injected mice with both HIV-infected human cells and with the reprogrammed naive CD8s into which the HIV-recognizing T-cell receptor genes had been inserted using a lentivirus delivery system. One week later when the researchers looked for HIV-infected human cells in the animals, they found that the infected cells had virtually been eliminated. Goldstein notes that this study was done using genes for just a single CD8 T-cell receptor. 'To make this strategy even more effective, we're now in the process of isolating a 'cocktail' of CD8 receptor genes that are specific for many different HIV peptides,' he says. 'Ultimately, we'd like to remove CD8s from patients, convert them into potent HIV-specific CD8s by inserting a variety of HIV-specific CD8 receptor genes, and then reinfuse them back into patients. By reinforcing the immune system in this way, we hope to turn the tide of battle against HIV in favor of people infected with the virus.'
From our Sponsors
Most Popular
Lexi Love comes out as HIV+ after Trump deletes federal resources
January 23 2025 11:23 AM
Ricky Martin delivers showstopping performance for 2024 World AIDS Day
December 05 2024 12:08 PM
Trump's orders prompt CDC to erase HIV resources
January 31 2025 5:29 PM
California confirms first case of even more deadly mpox strain
November 18 2024 3:02 PM
This long-term HIV survivor says testosterone therapy helped save his life.
December 16 2024 8:00 PM
Plus: Featured Video
Latest Stories
Grindr is reminding us why jockstraps are so sexy and iconic
May 02 2025 5:36 PM
Broadway's best raise over $1 million for LGBTQ+ and HIV causes
April 03 2025 7:15 PM
Season 4 of The Switch on resilience & radical self-love returns this spring
March 26 2025 12:20 PM
BREAKING NEWS: Trump admin moves to end federal HIV prevention programs
March 18 2025 6:10 PM
A camp for HIV-positive kids is for sale. Here's why its founder is celebrating
January 02 2025 12:21 PM
Decades of progress, uniting to fight HIV/AIDS
December 01 2024 12:30 PM
Climate change is disrupting access to HIV treatment
November 25 2024 11:05 AM
HRC holds 'die-in' to protest Trump health care cuts
April 28 2025 2:11 PM
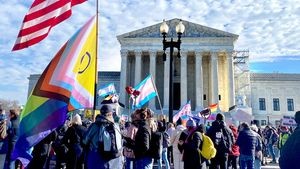
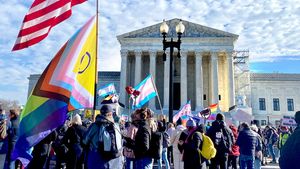
Two right-wing Supreme Court justices signal they may uphold access to PrEP and more
April 21 2025 4:10 PM
The Talk Season 5 premieres this spring with HIV guidance for the newly diagnosed
March 26 2025 1:00 PM
Jess King is here to help you live your happiest, healthiest life yet
March 24 2025 4:35 PM
Gerald Garth is keeping people of color happy and healthy through trying times
March 11 2025 3:38 PM
Tyler TerMeer vows to continue to fight for health care for all
January 28 2025 3:00 PM
'RuPaul's Drag Race' star Trinity K Bonet quietly comes out trans
December 15 2024 6:27 PM
500,000 Children at Risk: PEPFAR Funding Crisis
April 08 2025 3:51 PM
Discover the power of Wellness in your life
March 26 2025 12:41 PM
Celebrating Black History Month with our annual African American issue
February 01 2025 3:28 PM
Plus nominated for 2025 GLAAD Media Award
January 22 2025 12:42 PM
AIDS Memorial Quilt displayed at White House for the first time
December 02 2024 1:21 PM
Hollywood must do better on HIV representation
December 01 2024 9:00 AM
Trending stories
Recommended Stories for You