Treatment GuideJust DiagnosedSex & DatingAfrican AmericanStigmaAsk the HIV DocPrEP En EspañolNewsVoicesPrint IssueVideoOut 100
CONTACTCAREER OPPORTUNITIESADVERTISE WITH USPRIVACY POLICYPRIVACY PREFERENCESTERMS OF USELEGAL NOTICE
© 2025 Pride Publishing Inc.
All Rights reserved
All Rights reserved
By continuing to use our site, you agree to our Privacy Policy and Terms of Use.
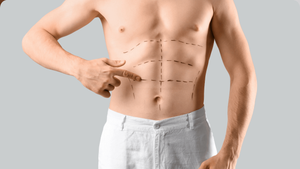
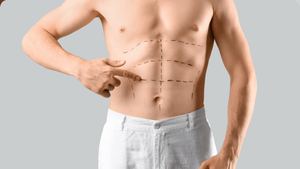
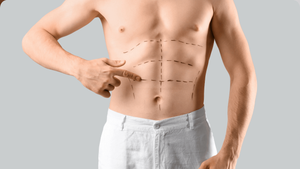
Lipodystrophy, the abnormal redistribution of body--fat stores, is found in 40% to 50% of U.S. HIVers, and it remains one of the disease's problematic outcomes. Its two principal components are lipoatrophy, the loss of fat in the limbs and face, and lipoaccumulation, the increase of fat within the abdomen and around the neck and chest. These may seem like opposite conditions, but both can occur in some patients. Initially protease inhibitors were seen as the cause of increased intra-abdominal fat, called visceral adipose tissue. We now know that this effect can be seen in patients who've never used protease inhibitors. With lipoatrophy there is a clearer association with the use of Zerit and AZT. But increased risk for either form of lipodystrophy includes duration and severity of HIV infection, lowest CD4 count, length of treatment, prolonged delay before treatment, and age. While lipoatrophy can be stigmatizing, it has no significant medical implications. Efforts to improve limb fat with insulin--sensitizing agents have had little success, but facial fillers can improve the appearance of people struggling with cheek--fat loss. Lipoaccumulation, though, is associated with insulin resistance, elevated lipids, and increased risk of heart disease. In extreme cases there can be painful abdominal distension and restricted breathing. Rigorous exercise and dieting'along with standard medications'can help reduce insulin resistance and lipid levels but have not had much effect on reducing intra-abdominal fat. Drugmakers had high hopes for two drugs as treatments for lipoaccumulation. Serostim was denied approval in July; but a new one is in the pipeline for approval. During studies of recombinant human growth hormone to treat HIV wasting, though, researchers have found some decrease in intra-abdominal fat. In one trial of recombinant human growth hormone (somatropin or Serostim), participants were given either a placebo or a four-milligram dose of growth hormone daily for 12 weeks. The group on Serostim was then reduced to a maintenance dosage of two milligrams every other day for another 24 weeks. After the first 12 weeks, intra-abdominal fat decreased 24% in the group taking recombinant human growth hormone. Mild side effects included edema and joint and muscle pain. Blood-sugar levels increased initially but then returned to normal. There was also loss of limb fat, which slowly returned to normal at the end of the 24--week maintenance period. On the every--other--day maintenance dose, about 40% of participants regained at least 50% of abdominal fat they had lost; this was considered a significant positive result. Because the surge of growth hormone that occurs in the body after it has been injected can have severe cardiovascular implications, such as disrupting glucose metabolism and elevating triglycerides, researchers have turned their attention to growth--hormone releasing factor. The advantage is that the releasing factor re--creates the natural pulsatile release of growth hormone and allows the regulatory feedback mechanism in the body to control the growth hormone effect. At this year's Conference on Retroviruses and Opportunistic Infections, Steven Grinspoon reported on a Phase III trial of TH9507, a synthetic growth hormone releasing factor made by Theratechnologies. At the end of a placebo--controlled 26--week trial with 412 participants, intra--abdominal fat decreased 15% in the group given TH9507 versus a 5% increase in the group who had received a placebo. Limb fat increased slightly in both groups. Better yet, the TH9507 group showed a decrease in total cholesterol and triglycerides and an increase in 'good' HDL cholesterol; in the placebo group triglycerides rose and HDL levels fell. In addition, there were no significant changes in insulin and glucose levels in the TH9507 group. Muscle aches and skin rash were the most common side effects. If synthetic growth hormone releasing factor eventually makes it to market, we may have our first victory in the battle of the bulge. Bowers is board-certified in family practice and is a senior partner with Pacific Oaks Medical Group, one of the largest U.S. practices devoted to HIV care.
From our Sponsors
Most Popular
“So much life to live”: Eric Nieves on thriving with HIV
September 03 2025 11:37 AM
Thanks to U=U, HIV-positive people can live long, happy, healthy lives
July 25 2025 2:37 PM
The Talk: Beyond the exam room
August 13 2025 3:15 PM
BREAKING: Supreme Court rules to save free access to preventive care, including PrEP
June 27 2025 10:32 AM
Messenger RNA could be the key to an HIV vaccine — but government cuts pose a threat
August 20 2025 8:02 AM
“I felt like a butterfly”: Niko Flowers on reclaiming life with HIV
July 23 2025 12:22 PM
Dancer. Healer. Survivor. DéShaun Armbrister is all of the above
July 02 2025 8:23 PM
The Talk: Starting the conversation
July 25 2025 4:47 PM
The lab coat just got queer
August 21 2025 10:00 AM
Plus: Featured Video
Latest Stories
HIV-positive men stage 'Kiss-In' protest at U.S.-Mexico border
December 01 2025 12:56 PM
What the AIDS crisis stole from Black gay men
December 01 2025 6:00 AM
Amazing People of 2025: Javier Muñoz
October 17 2025 7:35 PM
It’s National PrEP Day! Learn the latest about HIV prevention
October 10 2025 9:00 AM
“I am the steward of my ship”: John Gibson rewrites his HIV narrative
September 16 2025 2:56 PM
The Talk: Owning your voice
August 25 2025 8:16 PM
The Talk: Navigating your treatment
August 01 2025 6:02 PM
How the Black AIDS Institute continues to fill in the gaps
July 25 2025 1:06 PM
1985: the year the AIDS crisis finally broke through the silence
June 26 2025 11:24 AM
VIDEO: A man living with HIV discusses his journey to fatherhood
June 10 2025 4:58 PM
Trump admin guts $258 million in funding for HIV vaccine research
June 03 2025 3:47 PM
Grindr is reminding us why jockstraps are so sexy and iconic
May 02 2025 5:36 PM
HRC holds 'die-in' to protest Trump health care cuts
April 28 2025 2:11 PM
Two right-wing Supreme Court justices signal they may uphold access to PrEP and more
April 21 2025 4:10 PM
500,000 Children at Risk: PEPFAR Funding Crisis
April 08 2025 3:51 PM
Broadway's best raise over $1 million for LGBTQ+ and HIV causes
April 03 2025 7:15 PM
The Talk Season 5 premieres this spring with HIV guidance for the newly diagnosed
March 26 2025 1:00 PM
Trending stories
Recommended Stories for You