Treatment GuideJust DiagnosedSex & DatingAfrican AmericanStigmaAsk the HIV DocPrEP En EspañolNewsVoicesPrint IssueVideoOut 100
CONTACTCAREER OPPORTUNITIESADVERTISE WITH USPRIVACY POLICYPRIVACY PREFERENCESTERMS OF USELEGAL NOTICE
© 2026 Pride Publishing Inc.
All Rights reserved
All Rights reserved
By continuing to use our site, you agree to our Privacy Policy and Terms of Use.
The Food and Drug Administration has approved Aptivus, a nonpeptidic protease inhibitor from Boehringer-Ingelheim. The drug was shown to be effective against PI-resistant strains of HIV. Aptivus, now available nationwide, should be taken with a booster dose of Norvir. __________ Drugmaker Roche has advised health care professionals that it will stop sales and distribution of Fortovase, one of its protease inhibitors, by February 15 because of low demand. Roche's Invirase'which, like Fortovase, is a formulation of saquinavir'will continue to be available. __________ A pilot study in the July 1 edition of the Journal of Acquired Immune Deficiency Syndromes has shown that a nucleoside-sparing regimen containing just Kaletra and Sustiva is as effective as most three-drug regimens in reducing blood-based HIV viral loads and boosting CD4-cell counts. __________ HIV patients failing to achieve adequate blood-based concentrations when taking standard doses of the protease inhibitor Kaletra rarely achieve therapeutic levels by boosting their Kaletra dosage, according to a study in the July 1 edition of the journal AIDS. Drug side effects also were more likely with dosage increases. __________ A skin-patch test that screens for high levels of CD8 cells is effective in diagnosing risk factors for serious and possibly life-threatening hypersensitivity reactions to the nucleoside analog Ziagen. Researchers suggest combining the skin-patch test with immunological and genetic screenings to help diagnose and prevent Abacavir Hypersensitivity Syndrome. __________ Pfizer has announced it is abandoning development of experimental nonnucleoside analog capravirine because tests showed it was not more effective than existing antiretroviral drugs. __________ Tibotec is launching an expanded access program for the experimental protease inhibitor TMC114. Tests have shown that TMC114 is active against protease-resistant strains of HIV. Tibotec says that in 2006 it will seek accelerated approval for the drug. __________ Gilead Sciences has launched a Phase I/II study of its experimental integrase inhibitor GS 9137, which aims to prevent HIV from integrating viral genetic material into cellular DNA, a step necessary for the virus to replicate. The drug was previously evaluated in a Phase I trial in Japan. __________ A study in the July edition of The Annals of Pharmacotherapy has shown that once-daily administration of Ziagen is as effective in suppressing HIV replication as a twice-daily dosing, but the dose was linked with increased risk for hypersensitivity reactions and severe diarrhea. __________ Bristol-Myers Squibb has cut the price in poor nations of pediatric formulations of Zerit and Videx. Zerit's price was reduced by 44%; Videx's price was cut by 90%.
From our Sponsors
Most Popular
“So much life to live”: Eric Nieves on thriving with HIV
September 03 2025 11:37 AM
The Talk: Beyond the exam room
August 13 2025 3:15 PM
Messenger RNA could be the key to an HIV vaccine — but government cuts pose a threat
August 20 2025 8:02 AM
It’s National PrEP Day! Learn the latest about HIV prevention
October 10 2025 9:00 AM
Amazing People of 2025: Javier Muñoz
October 17 2025 7:35 PM
The lab coat just got queer
August 21 2025 10:00 AM
The Talk: Owning your voice
August 25 2025 8:16 PM
“I am the steward of my ship”: John Gibson rewrites his HIV narrative
September 16 2025 2:56 PM
Plus: Featured Video
Latest Stories
HIV-positive men stage 'Kiss-In' protest at U.S.-Mexico border
December 01 2025 12:56 PM
What the AIDS crisis stole from Black gay men
December 01 2025 6:00 AM
The Talk: Navigating your treatment
August 01 2025 6:02 PM
The Talk: Starting the conversation
July 25 2025 4:47 PM
Thanks to U=U, HIV-positive people can live long, happy, healthy lives
July 25 2025 2:37 PM
How the Black AIDS Institute continues to fill in the gaps
July 25 2025 1:06 PM
“I felt like a butterfly”: Niko Flowers on reclaiming life with HIV
July 23 2025 12:22 PM
Dancer. Healer. Survivor. DéShaun Armbrister is all of the above
July 02 2025 8:23 PM
BREAKING: Supreme Court rules to save free access to preventive care, including PrEP
June 27 2025 10:32 AM
1985: the year the AIDS crisis finally broke through the silence
June 26 2025 11:24 AM
VIDEO: A man living with HIV discusses his journey to fatherhood
June 10 2025 4:58 PM
Trump admin guts $258 million in funding for HIV vaccine research
June 03 2025 3:47 PM
Grindr is reminding us why jockstraps are so sexy and iconic
May 02 2025 5:36 PM
HRC holds 'die-in' to protest Trump health care cuts
April 28 2025 2:11 PM
Two right-wing Supreme Court justices signal they may uphold access to PrEP and more
April 21 2025 4:10 PM
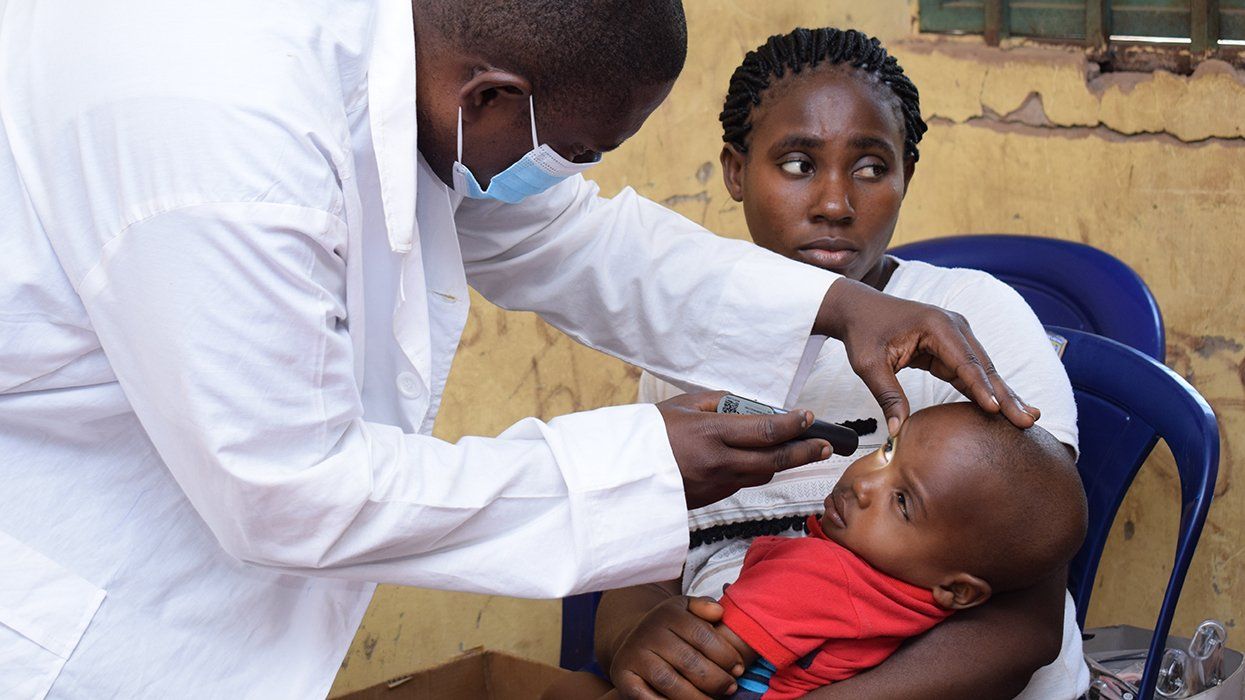
500,000 Children at Risk: PEPFAR Funding Crisis
April 08 2025 3:51 PM