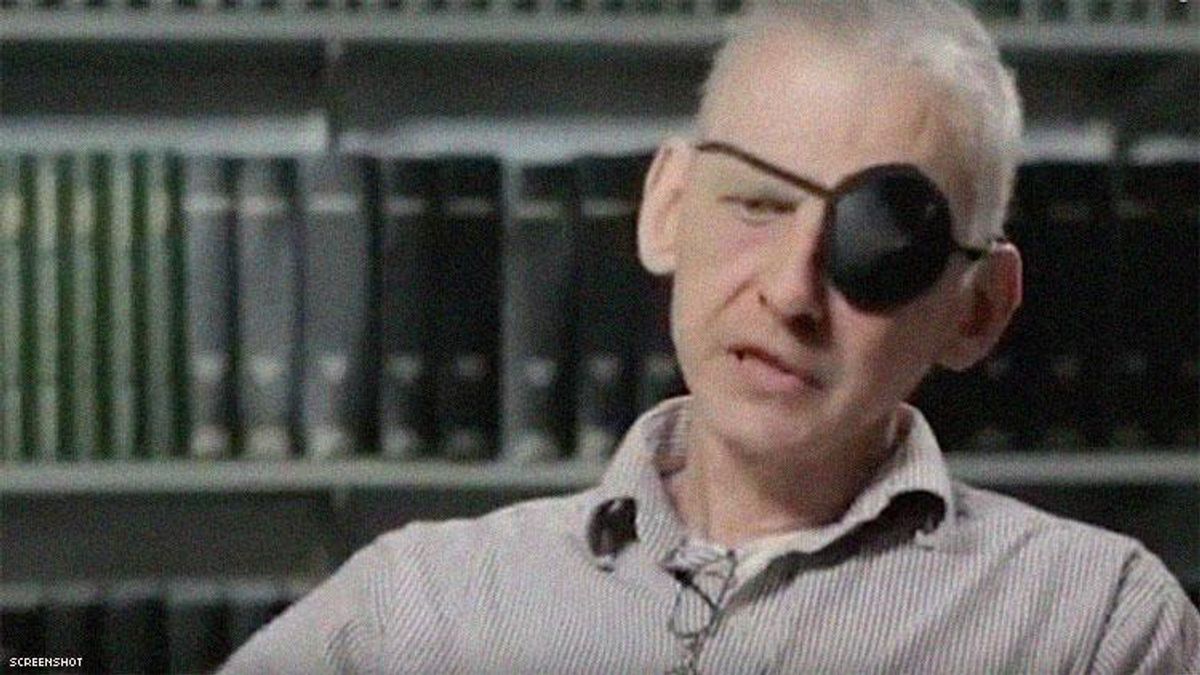
SIU’s ethics panel launched a “full” investigation Dec. 5 of the herpes vaccine experiments by university professor William Halford, according to a memo obtained by Kaiser Health News.
Halford, who died in June, had injected Americans with his experimental herpes vaccine in St. Kitts and Nevis in 2016 and in Illinois hotel rooms in 2013 without routine safety oversight from the Food and Drug Administration or an institutional review board, according to ongoing reporting by KHN. Some of the participants say they are experiencing side effects.
The panel, known as the Misconduct in Science Committee, told SIU’s medical school dean that the inquiry should not only investigate the extent of Halford’s alleged wrongdoing, but also scrutinize “members of his research team,” according to the Dec. 5 memo obtained through a Freedom of Information Act request.
“The Misconduct in Science Committee is now in its investigative stage and the School anticipates this investigation will take approximately 120 days,” SIU spokeswoman Karen Carlson told KHN in an emailed response. “However, the investigation could take longer.”
The panel’s inquiry marks the second one to be launched by SIU since Halford’s methods were detailed in a KHN report in 2017.
The Department of Health and Human Services asked the university to determine whether Halford’s activities violated the institution’s pledge to HHS. SIU, a state university, had pledged to follow human-subject safety protocols for all research, even if privately funded.