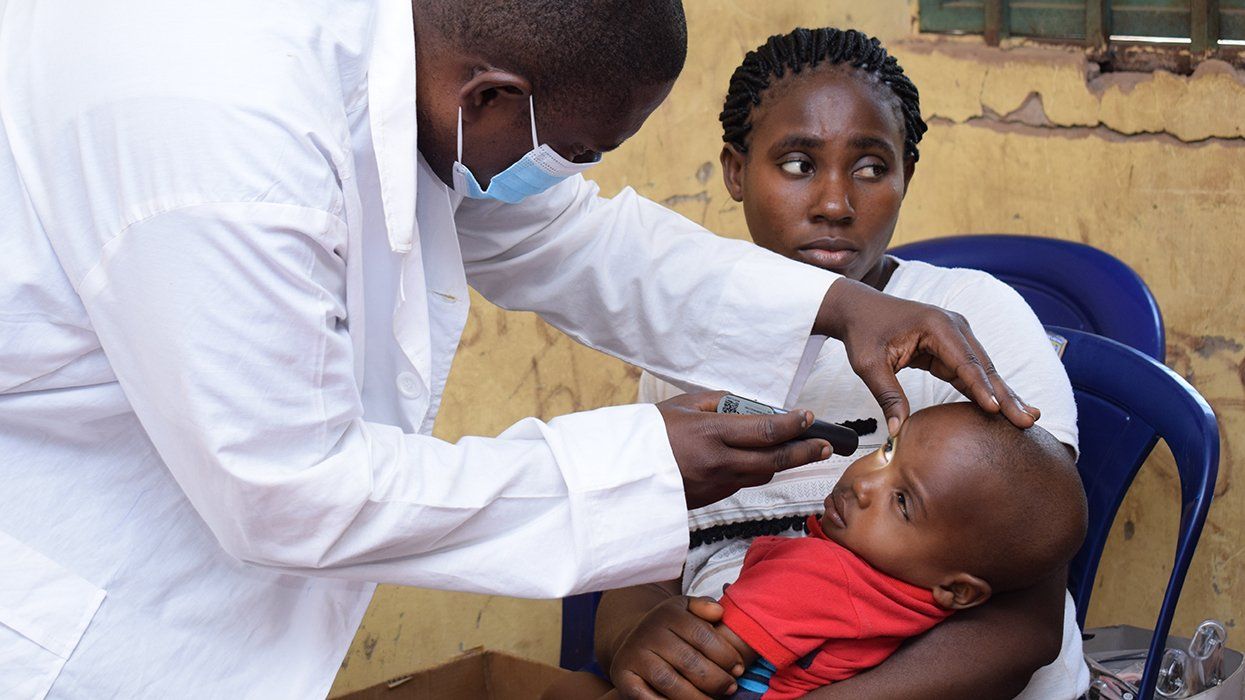
Tivicay, the recently approved HIV integrase inhibitor — a class of antiretroviral drug that is designed to block a virus from entering the DNA of a host cell — demonstrated high rates of viral suppression in a recent study. Significantly, Tivicay is also proven effective for people who are resistant to HIV antiretroviral drugs, according to NAM.
The study concluded that among people starting treatment for the first time, there was no resistance detected during the 96 weeks of follow up, according to findings presented at the recent 20th International AIDS Conference in Melbourne, Australia.
Modern antiretroviral treatments are highly effectively and are well-tolerated. However some people who have resistance to the existing drugs or may have difficulty tolerating specific side-effects.
Jim Demarest of ViiV Healthcare lead a team that analyzed the outcomes among participants that were part of phase three trials with Tivicay. Three studies followed people who had not previously taken any HIV medications, while one trial focused on those who have received treatment before and had experienced resistance to two or more drug classes.
In the studies that followed treatment-experienced participants, 71 percent of participants that took Tivicay saw viral suppression at 48 weeks, compared to the 64 percent of those who took Isentress, another drug used to treat HIV.
Regulators in the U.S. are currently evaluating a fixed-dose medication containing Tivicay and Kivexa or Epzicom. If the drug is approved, it will be the first one-pill, once-daily regimen that does not contain tenofovir DF (brand name, Viread), which some people with HIV have avoided because of its risk of kidney and bone toxicity. This combination has already received approval by the European Medicines Agency and will be marketed as Triumeq in Europe.