Treatment
Long-Acting Injectable Shows Promise In First Human Trials
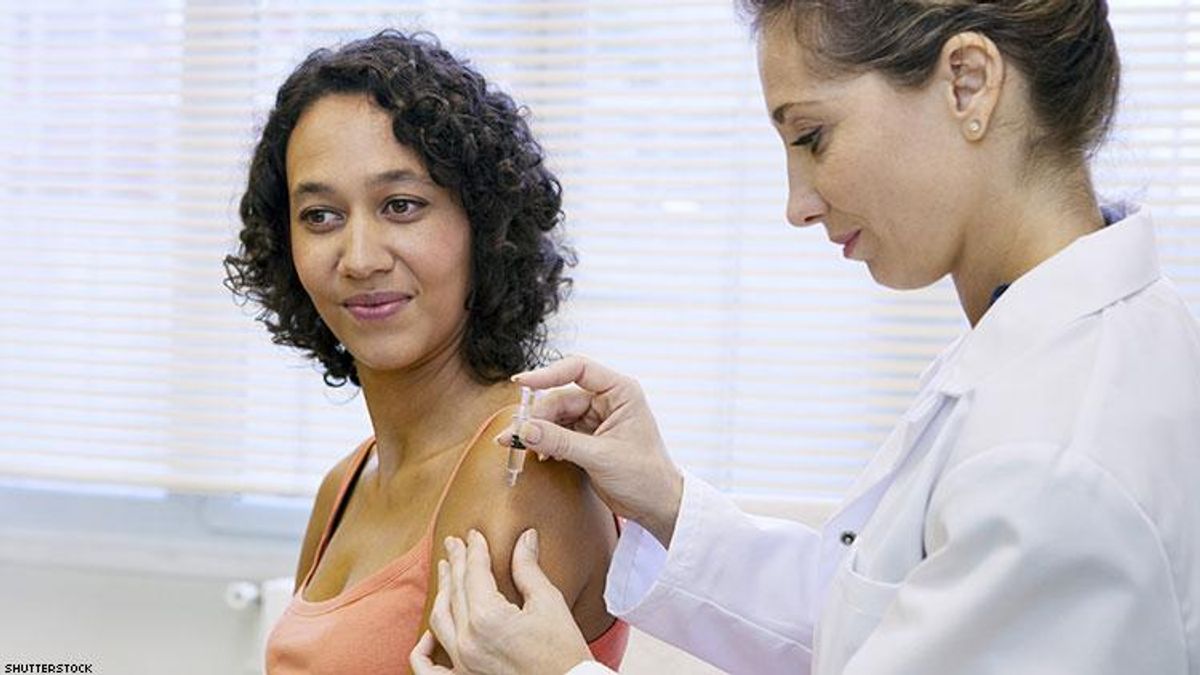
Researchers say that GS-6207, Gilead's first-in-class capside inhibitor, can remain in our body's system for up to 24 weeks.
David Artavia
Editor
March 12 2019 2:38 PM EST
By continuing to use our site, you agree to our Privacy Policy and Terms of Use.
Researchers say that GS-6207, Gilead's first-in-class capside inhibitor, can remain in our body's system for up to 24 weeks.
According to results from an early clinical trial presented at the Conference on Retroviruses and Opportunistic Infections (CROI 2019) last week in Seattle, a new long-lasting injectable appeared safe when administered to humans.
If all goes according to plan, researchers say the injectable could potentially be administered every three months or less.
The new drug is called GS-6207, Gilead’s first-in-class capsid inhibitor. As NAM AIDS Map reports, Jennifer Sager of Gilead Sciences presented findings from a phase I study — the first in humans — evaluating the safety and pharmacokinetics of the new drug in HIV-negative volunteers.
While modern antiretroviral therapies are generally tolerated well, newer medications have shown to offer different avenues in treating and preventing HIV, especially for poz folks whose virus can be resistant to certain types of drugs. Long-acting therapies like GS-6207 could potentially improve adherence and help make life easier for those needing to take multiple medications every single day.
GS-6207 works by interfering with the assembly and disassembly of the HIV capsid, which is basically the genetic blueprint of the virus.
In essence, GS-6207 acts at multiple stages of the HIV replication cycle by interfering first with disassembly of the capsid and transport of viral genetic material into a host cell's nucleus, as NAM AIDS Map reports, then later with assembly of capsids for newly produced virus, which results in immature viral particles that cannot infect other cells.
Sager presented at CROI 2019 that during the human trial (all of whom were HIV-negative), a single injection of GS-6207 resulted in sustained concentrations for at least 24 weeks. Using 300 and 450mg doses, estimated drug levels at week 12 remained well above the 95% effective concentration.
The fact that the drug itself can remain in the system for up to 24 weeks should give hope to possible long-lasting injections for HIV-positive people.
"The PK profile of subcutaneously administered GS-6207 is consistent with sustained delivery, supporting a dosing interval of at least three months at doses greater than 100mg," Gilead said in a press release.
To give a bit more context, GS-6207 is a refined version of a investigational capsid inhibitor called GS-CA1, which was presented at CROI 2017.
GS-CA1was highly potent at low doses against multiple HIV-1 isolates and appeared well tolerated with no measurable cell toxicity, and showed success in early studies. But after some refinements, scientists eventually created a smaller version of it (GS-6207), whic they used for human testing.
There have been no serious adverse events or deaths and no severe laboratory abnormalities deemed to be of clinical relevance so far, stated Sager. All adverse events seen so far have been mild or moderate (grade 1 or 2) with no clear pattern according to dose. The most common side effect was mild and transient injection site reactions.
That being said, a phase Ib proof-of-concept study is now underway to be administered to HIV-positive people. And, according to Sager, researchers are now working to develop an oral formulation of GS-6207.