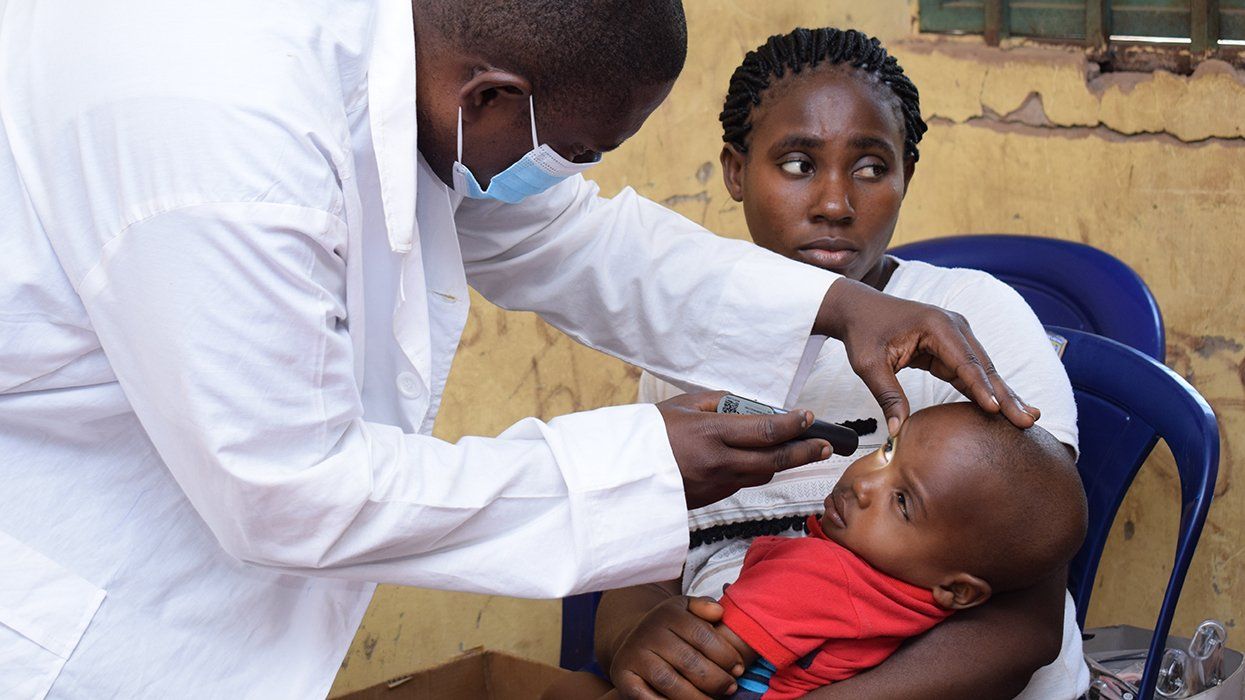
Gilead Sciences recently released new research data reinforcing and further proving that Biktarvy is a highly effective treatment option for a broad range of people living with HIV, including those also dealing with a hepatitis B diagnosis (HBV).
Interim data from the Alliance trial showed that Biktarvy (which comes in tablets made up of 50 mg of bictegravir, 200 mg of emtricitabine, and 25 mg of tenofovir alafenamide) did a better job than an alternative treatment of suppressing hepatitis B virus in people who are living with both HIV and HBV.
Additional data from two Phase 3 trials further demonstrated Biktarvy’s sustained efficacy, safety, and high barrier to resistance in adults with HIV who are just starting treatment. The findings were presented in July at the 24th International AIDS Conference.
A press release detailing the new data noted that “HIV/HBV coinfection is a major global public health threat that increases the morbidity and mortality beyond either infection alone. HBV impacts approximately 8 percent of people with HIV globally, and HIV/HBV coinfection rates can reach 25 percent in areas where both viruses are endemic, such as Asia.”
“Alliance is a landmark clinical trial, investigating the specific treatment responses of adults with HIV/HBV coinfection,” said Dr. Anchalee Avihingsanon, a senior researcher who works with the Thai Red Cross AIDS Research Center in Thailand. “Emerging HIV epidemics in areas of high HBV rates such as Asia are expanding the number of people with HIV/HBV coinfection. This inclusive and representative study enrolled and treated participants from 11 different geographies with 88 percent of participants of Asian descent, driving the availability of data from within those communities most impacted.”
In addition to these findings, Gilead presented other new data at AIDS 2022. Company officials shared five-year cumulative data that demonstrated Biktarvy’s sustained efficacy and durable viral suppression as first-line therapy in people with HIV. Not one case of treatment failure occurred in an analysis of five years of data from both studies, which further demonstrates the effectiveness and anti-resistance properties of Biktarvy for the treatment of HIV in adults with no prior antiretroviral therapy history.
Additionally, pooled data showed that 99 percent of participants who initiated treatment with Biktarvy and remained in the study for all 240 weeks achieved and maintained an undetectable viral load through five years of follow-up. The data supports showed long-term use of Biktarvy caused no significant changes to metabolic, bone, and renal markers.
“As we strive to optimize HIV treatment and advance scientific innovation, we’re committed to tailoring our research to address the individual needs of all people with HIV, including those with comorbidities,” said Dr. Jared Baeten, vice president of HIV Clinical Development at Gilead. “The HIV treatment research data presented at the 24th International AIDS Conference are an important step in deepening our understanding of how to support the long-term and overall health of a broad range of people with HIV worldwide.”